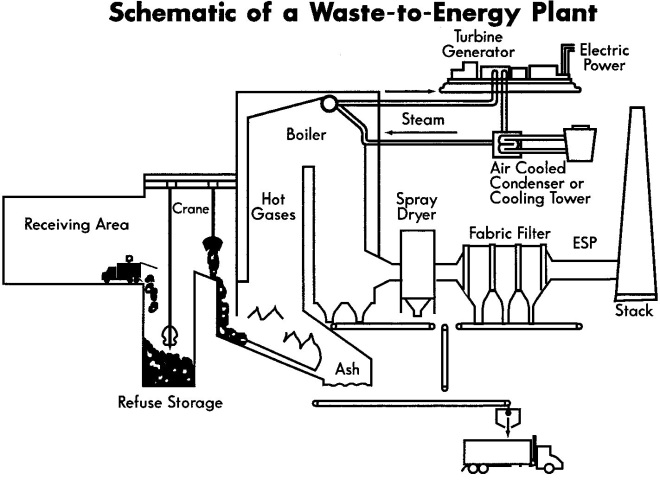
Waste is a big problem in Jakarta. This city produces approximately 30 – 37 thousand meter cubic or 7700 tons per day domestic waste which 15% is unable to be collected and removed to the primary landfill. There is no reference detailing the percentage composition of degradable and non-degradable, but the important thing I want to highlight here is the absence of long term strategy to manage non-degradable waste. While in developed countries such as Canada, France and Sweden, the regional governments realize that the demand of waste management and waste incineration technology are urgent. Waste incinerator has an important role, not only to decompose non-degradable waste but also to convert it into heat energy which can be reused for electricity.
However, waste incineration technology is still posing a lot of technical problems due to corrosion phenomena. High Temperature Corrosion (HTC) in waste incinerator is caused by chlorine attack, in which its fundamental corrosion mechanism is not well understood. This mechanism is slightly different with that of HTC in gas turbine, diesel engine or furnace where corrosion is induced by the presence of hot gases containing Vanadium or Sulfates.
One of the leading research group which conduct extensive research in this field is Swedish High Temperature Corrosion group. I was lucky because I have visited this research group in Goteborg a year ago. Their laboratories are well equipped, having strong financial support from local renewable energy companies and they were really interested to my internship paper about HTC in incineration facility. This is the reason that I want to publish it so everyone can understand the recent problem and what are the primary concerns in the coating technique selection in waste incineration.
I. INTRODUCTION
Waste management is in crisis in the world largest urban areas as the population of the cities continues to grow. This has led to the high demand of waste incineration which can accommodate the increasing of solid waste in very limited disposal space. The integration of waste incineration and energy recovery, well known as Waste to Energy (WTE) is believed to be beneficial since it offers not only volume reduction of waste (until 90%) adaptable to any kind of waste (municipal to biologic pollutant) but also releases combustion energy which can be transformed to heat and electricity form and eventually gas fuel.
Chlorine containing corrosion in high temperature is a challenge that crosscuts WTE industries and is considered as the worst condition in corrosion case. Some studies have shown that chlorine corrosion is a major problem in WTE and biomass-fired boiler since chlorine gaseous (560-2240 vppm) was dominant over other compound such as sulfur (100-2000 vppm). Chlorine concentration in fuel were 0.5% in average, or 5 times higher than that of coal power stations which allow them to be operated in higher temperature and thus have better efficiency (up to 33%).
In active oxidation, corrosion rate is appeared to be proportional to temperature, whereas reducing the temperature of steam is undesirable in reason of thermal efficiency. It is clear that the efficiency of plant is limited by corrosion resistance of materials. Therefore, coating method with special material was developed to obtain material which could withstand in very aggressive environment at high temperature. Some coating materials with certain deposition methods were successfully developed in laboratory scale but failed in industrial application or were not economically viable at large scale. Therefore, if some methods are promising, further investigations are still needed.
Heat transfer surfaces like waterwall, screen tubes and the superheater are the most sensitive areas attacked by corrosion. Heat exchanger (Waterwall) and superheater tube are very risky to chlorine corrosion attack in WTE due to the presence of chlorine containing deposit as explained on the following section.
The mechanism of chlorine corrosion attack is still debated. However, two well known mechanisms were proposed:
1. Corrosion due to active oxidation
It is possible that direct gaseous corrosion attack leading to active oxidation of metal substrate Fe2O3 which has spongy non protective surface. This reaction is also releasing Cl2 gaseous which may diffuse back into the surface and promote again the formation of volatile metal chlorides. However, some works confirmed that gaseous chlorine attack is less corrosive than alkali chloride condensed on superheater surface
2. Corrosion due to deposit by sulfation and molten salts
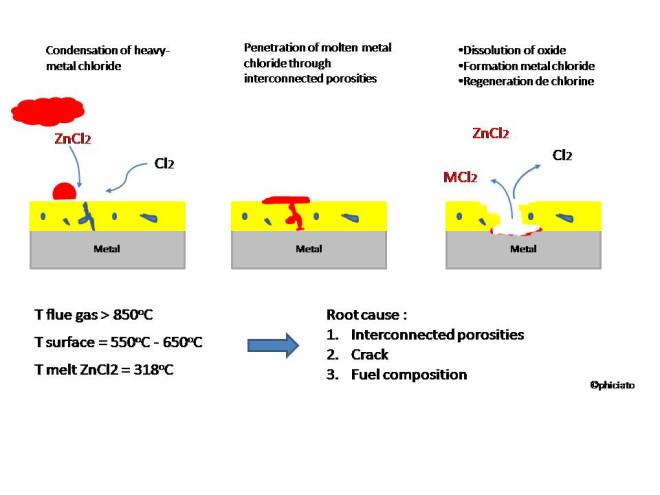
This corrosion mode is also known as fluxing mechanism since the product of this reaction may dissolve protective oxide scale and accelerate loss of coating when heavy-metal chlorides such as PbCl2, ZnCl2 present (see illustration below). Some publications suggested that only one mechanism is involved while others suggested that the mechanism of corrosion is a combination of both: direct gaseous attack and deposit by sulfation of molten salts. When the chlorinated gas penetrates and reaches the metal surface M, it will immediately react with M and forms an eutectic metal chloride M-Cl which is volatile below 900oC, while the temperature of flue gas on the surface may reach more than 850oC.
II. COATING DEVELOPMENT FOR WASTE INCINERATION PLANTS
Nowadays, the two main routes that are explored for fighting corrosion in WTE and more particularly in superheaters and heat exchangers are (i) the development of new corrosion-resistant alloys and composites that can withstand the high temperatures necessary to attain a high efficiency level and (ii) the development of corrosion-resistant coatings. This work focuses on the second route. The substrate and coating materials characteristics are reviewed.
2.1 Substrate material
Superheater and heat exchanger are the most risky parts to corrosion attack in WTEs due to condensation of volatile metal chloride as already explained in section I. Important considerations for the selection of substrate material for superheaters and heat exchangers are thermal conductivity and material cost. Substrate materials should have high thermal conductivity as well as high corrosion resistance to perform well at high temperatures. Ferritic and austenitic steels are the most common metals used as substrate material. Experimental results showed that chromium content in typical ferritic steels 2.25 w.%t results in good conductive properties and low cost. While austenitic steels chromium contained higher amount of chromium (27 wt.%), nickel (31 wt.%) are more resistant to corrosion but less thermally conductive and also more expensive. In boiler design, austenitic steels are used only if ferritic steels cannot withstand the high temperatures of operation, the mechanical load or the corrosive environment.
2.2 Coating materials and methods
Theoretical specifications of coating which are described in details in this reference suggested that coatings should be thermodynamically stable, show minimum interdiffusion and their Coefficient of Thermal Expansion (CTE) should be as close as possible to that of substrate. However, there are two other additional specifications that should be considered as complementary factors.
1. Coating should show minimum dissolution induced by melting of metal chloride. In highly chlorinated environment like waste incineration, oxide dissolution that is is well known as fluxing mechanism is an initial step in corrosion mechanism. Fluxing is referred to dissolution of oxide layer that is protecting metal surface caused by deposit of low melting point salt. P.Viklund found that in ferrous alloys with Ni, Cr, Mo, the addition of Nickel and Molybdenum were beneficial even though they do not contribute to the formation of protective oxide scales but Ni may form NiO which is less soluble in chloride salt.
2. Coating should be thick enough with no interconnected porosity. If crack occurs on surface, thick coating may postpone the diffusion of corrosive substances until the underneath layer forms protective oxide, thus increasing lifetime of service.
According to the mechanism of corrosion described in section I, it is clear that permeability of protective oxide is responsible for all of corrosion attack modes. The direct chlorine gaseous attack and metal chloride cycle attack induced by the formation of metal chloride can be avoided if only the corrosive species are unable to penetrate and to reach the metal substrate. Thus, it can be concluded that coating should be designed as impermeable as possible by reducing interconnected porosity and by improving adherence between substrate and coating.
Various coating methods were developed and two types of coating can be generally distinguished: traditional plain coating composed by a single layer and multi layer coatings with an intermediate self healing barrier. Details of advantages and disadvantages of each techniques are resumed on the table below.